Introduction to Thermodynamics
What is Thermodynamics?
Thermodynamics is the branch of science which deals with the relations among heat, work, and properties of the system which are in equilibrium. It describes state and changes in the state of physical systems. The word Thermodynamics is got from the Greek words. Thermos means Heat and Dynamics meaning flow. Thermodynamics deals with heat and work.
It is based upon observations of common knowledge that have been formulated into thermodynamic law.
The applications of the thermodynamics law :
- Energy Technology
- Notably in steam
- Nuclear power plants
- Internal Combustion engines
- Compressor
- Chemical process plants
- Direct energy conversions
Terminologies used in Thermodynamics
A system is a finite quantity of matter under consideration that is separated from the rest of the universe by real or imaginary boundaries.
 |
| (Thermodynamic terminologies) |
Everything in the universe that is not part of the system and can interact with it is called as surroundings. That is outside of the system.
Boundary defined as it is (fixed or movable) separate the system from the surroundings. The boundary is sometimes real sometimes imaginary.
For example, take the cricket stadium here cricket ground is a system, outside the cricket stadium is surroundings and the wall or place which the audience sat is a separate system from the surroundings is called Boundary.
Types of Systems
- Closed system
- Open system
- Isolated System
Closed System
A system that permits the exchange of energy but not masses, across the boundary with its surroundings is called a closed system.
An example of this system is a mass of gas contained in an engine, a closed beaker, and a Pressure cooker.
Open System
A system that permits the exchange of energy and mass, across the boundary with its surroundings is called an Open system. Most of the engineering systems are always open.
An example of this system is the turbine, boiler, steam engine, and open beaker.
Isolated System
An isolated system is defined as a system that can exchange neither mass nor energy with its surroundings.
Eg. Universe
Till now we didn't find anything is around the universe so it is called an Isolated system.
 |
| (TYPES OF SYSTEMS) |
Homogeneous system
A quality of matter homogeneous throughout in chemical composition and physical structure is called a phase. A system containing a single-phase is called a homogeneous system.
Eg. A Mixture of Nitrogen and Oxygen.
Heterogeneous systems
A system containing more than one phase is known as a heterogeneous system.
Eg. Ice floats in water.
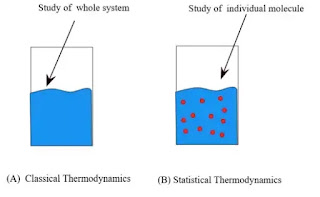
Macroscopic view vs Microscopic view
Macroscopic View | Microscopic View |
In this approach to thermodynamics is concerned with gross or overall behavior. | In this approach, a certain amount of matter is considered the events occurring at the molecular level. |
Simple Mathematical formulae required | Advanced Mathematical and statistical formulae are required. |
To Describe the few properties required | To Describe a large number of variables are needed |
The properties are easily measured by instruments. | The properties cannot be easily measured by instruments |
It is also called as Classical Thermodynamics | It is also called as Statistical Thermodynamics |
 |
| (Macro and microscopic studies) |
Properties of Systems
A property of a system is a characteristic of the system which depends upon its state, but independent of how the state is reached.
Intensive properties
These properties are free from the mass of the system.
Eg. Pressure and Temperature.
Extensive properties
These properties free from the mass of the system.
Eg. Volume
If we divide Extensive properties by mass associated with them to obtain the intensive properties.
If Specific Volume is called intensive property.
State
The state defined as the condition of the system at an instant of time as described or measured by its properties also state is defined as each unique condition of a system.
Thermodynamic Equilibrium
A system that satisfies the conditions of thermal, mechanical, and chemical equilibria and contains the macroscopic properties which are independent of time is said to be in thermodynamic equilibrium.
Thermal Equilibrium
The temperature of the system does not vary concerning time and temperature the same at all points.
Mechanical Equilibrium
The pressure in the system is constant at all points and does not change concerning time. The unbalanced force, not in the system.
Chemical Equilibrium
Chemical composition which is the same through the system does not vary with time and No chemical reaction takes place in the system.
Thermodynamic Process
If the system undergoes a change in state or an energy transfer at a steady-state the process has occurred.
Non-flow process
A process may non-flow in which a fixed mass within the defined boundary is undergoing a change of state. The closed system undergoes non-flow processes.
Eg. Heating the substance within the cylinder.
Flow process
A process may a flow process in which mass is entering and leaving through the boundary of an open system.
Quasi-static process
Quasi means almost. A quasi-static process is also named a reversible process. This process is a succession of equilibrium states and unbounded slowness is its characteristic feature. A process which is but a locus of all equilibrium points passed by the system.
Different types of processes that are commonly used in the study of thermodynamics.
Isothermal Process
The isothermal process is defined as one in which the temperature of the system remains constant during the change from its initial to final states. During the isothermal process, the system allows to exchange of heat with its surroundings and the temperature of the system remains constant.
Adiabatic Process
An adiabatic process is defined as that one that does not exchange heat with its surroundings during the change from initial to final states of the system. A thermally and completely insulated system with its surroundings can have changes in temperature during the transformation from initial to final states in an adiabatic process.
Isobaric or Constant Pressure Process
The isobaric process is that process in which the pressure of the system remains constant during its change from the initial to the final state.
Isochoric or Constant Volume process
The isochoric process shows no change in the volume of the system during its change from the initial to the final stage of the process.
Reversible process
In a reversible process, the series of changes carried out on the system during its transformation from initial to the final state may be possibly reversed exactly. This is possible when the changes are carried out very slowly in many smaller steps on the system during its change from initial to the final state. By doing so, each of its intermediate states will be in equilibrium with its surroundings.
For example, when the ice melts a certain amount of heat is absorbed. If we remove the same amount of heat to form water, we will get ice.
Irreversible Process
An irreversible process is one that cannot be retained to the initial state without making a permanent change in the surroundings. Spontaneous processes are always irreversible in nature.
 |
| (Reversible and irreversible) |
Reversible Process | Irreversible Process |
This process is slow when it is going through a series of smaller stages with each stage maintaining equilibrium between the system and surroundings. | In this process, the system reaches the final state from the initial state with an observable speed. During the conversion, there is no equilibrium maintained between the system and surroundings. |
The reversible process occurs in the forward and backward direction | The irreversible process occurs only one direction |
Work done is high compare to the irreversible process | Work done is small compare to the irreversible process |
If we brought back the reversible process, we need not change anything in the surrounding. Eg.Exapansion and compression of elastic material like spring, rubber, etc. Electrolysis | If we brought back the reversible process, we need to change anything in the surrounding. Eg. Relative motion with friction Diffusion Throttling |
Cycle
A cycle is defined as the series of processes the end states are the same.
For example, we are going to college or school every day. First, we will go to the school from the house and after school time finished we will come to our home. This scenario is called a cycle.
Point function is defined as two properties that are located a point on the graph (co-ordinate axes) then those properties are called point function.
Eg. Pressure, Volume, Temperature.
The path function is defined as two properties that are not located a point on the graph then those properties are called path function.
Eg. Heat and Work.
The temperature is a thermal state of a body that differentiates a hot body from a cold body.
The temperature is directly proportional to the molecular energy stored in the body.
There are various types of instruments are available to measure the temperature of the body.
For ordinary temperature we can use a thermometer and to measure high temperature we will use Pyrometers.
The SI unit of temperature is Kelvin(K). We can also measure temperature in °C( Degree Celsius) 1°C =273.15 K.
Force per unit area is called pressure. The pressure may be caused by gases, vapors, and liquids. The instrument that is measuring the only gauge pressure that means how much amount of pressure caused by gases, vapors, and liquids. That instrument didn't take the atmospheric pressure (Pressured exerted by the atmosphere ).
When gauge pressure is greater than atmospheric pressure is known as positive pressure.
When gauge pressure is less than atmospheric pressure is known as vacuum pressure.
Absolute pressure means the sum of gauge pressure and atmospheric pressure.
- Absolute pressure= Atmospheric pressure + Gague pressure
- Vacuum pressure = Atmospheric pressure - Absolute pressure.
Atmospheric pressure is measured by the barometer.
Unit of pressure: SI unit is N/m2 or we can call pascal (Pa). Another term is bar, 1 bar = 105 N/m2.
Standard atmospheric pressure is = 1.01325 bar that is equal to 760 mm of Hg (Hg means Mercury).
Work is defined as force moves through a system. Also, we can say the product of the pressure and area. Typically it is defined as it is a transient quantity that only appears at the boundary while the displacement of the state is taking place within a system.
- Work done by the system is termed as positive work. Eg. When a gas expands pushing a piston outwards.
- Work done on the system is termed as negative work. Eg. Force applied to a rotating handle.
The form of energy that is transferred across a boundary by virtue of a temperature difference is known as heat.
- Heat received by the system is Positive heat (+Q).
- Heat rejected by the system is Negative heat (-Q).
Heat | Work |
To heat transfer temperature difference is required | The temperature difference is not required |
Stable system attain the heat transfer | Work is cannot be transferred by a stable system |
The external effect is calculated in heat transfer | The external effect is not calculated in work transfer |
Similarities: | |
Heat and work are path functions inexact differentials. | |
Heat and work are correlated with a process, not a state | |
The system possesses energy but not in the form of work or heat. | |
The amount of heat is required to raise the temperature of the substance is known as sensible heat.
If we applying heat is sensible, the temperature of the system varies.
The amount of heat is required to change the phase of the substance is known as latent heat.
The temperature of the system doesn't vary while phase changing.
For example,
If we heat the water, the temperature of the heat increasing until the water vaporization takes place this heat is known as sensible heat and if we add heat during the vaporization, phase change occurs then the temperature of the water is constant that means latent heat.
It is defined as the amount of heat required to raise a unit mass of the substance through a unit rise in temperature. the symbol is used to mention specific heat C.
SI unit of the specific heat is J/kg K.
 |
| (Graphical representation) |
Conclusion
That's all about the Basics of Engineering Thermodynamics, I hope you understand the definitions, types, terminologies of Thermodynamics.
If you have any queries or doubts or need improvements in this article put that in the below-mentioned comments section we will reply and rectify as fast as possible.
