Zeroth law of Thermodynamics
 |
Hello mechons, in this post we are going to explain to you about a little law of the thermodynamics called Thermodynamics Zeroth law and also the example of zeroth of Thermodynamics.
Ok first, let me start with
What is the Zeroth Law of Thermodynamics?
According to the statement of "Zeroth law of Thermodynamics",
When a body X is in thermal equilibrium with a body Y, and also with a body Z, then Y and Z will be in thermal equilibrium with each other.
All the temperature measuring types of equipments and instruments are working under the zeroth law of thermodynamics.
Before we going to see the examples just read the basics of Thermodynamics article from us so, that you can get clear knowledge in the upcoming topic.
Examples of Zeroth law of Thermodynamics
Let us consider one example measuring of the temperature of the body. If we place the thermometer in the mouth that is body X and Thermometer class contact with the body that is body Y thermometer which consists of mercury that is known as a body Z at a certain time three bodies come in thermal equilibrium with each other.
Thermometer:
To obtain a quantitative measure of temperature, a reference body or datum is used, and a certain physical characteristic of this body which varies with temperature is selected. The changes in the selected characteristic may be taken as an indication of varies in temperature. The selected characteristic is called the thermometric property, and the reference or datum body which is used in the determination of temperature is called the thermometer. Most commonly thermometer consists of a small amount of mercury in an evacuated capillary tube.
Measurement of Temperature-The reference Points
If X is the thermometric property, we can arbitrarily choose for the temperature common to the thermometer and to all systems in thermal equilibrium with it the following linear function of :
⍬(X) = aX, where a is an arbitrary constant.
If X1 corresponds to ⍬(X1), then X2 will correspond to
[⍬(X1)/X2] X2
that is ⍬(X2)= [⍬(X1)/X2] X2
Two temperature on the linear X scale is to each other as the ratio of corresponding Xs.
Thermometer | Thermometric property | Symbol |
1. Constant volume gas thermometer | Pressure | p |
2. Constant gas thermometer | Volume | V |
3. Electrical resistance thermometer | Resistance | R |
4. Thermocouple | Thermal e.m.f | ɛ |
5. Mercury-in-glass thermometer | Length | L |
Electrical Resistance Thermometer
The resistance of the thermometer the change in resistance of a metal wire due to its temperature change. The wire, frequently platinum, may be incorporated in a Wheatstone bridge circuit.
The platinum resistance thermometer can measures temperature high degree of accuracy and sensitivity, this element is used for standard and calibration of other thermometers.
Restricted range
R = Ro (1+At+B t2)
Ro - the resistance of the platinum wire. A & B are constants.
 |
| (Electrical Resistance Thermometer) |
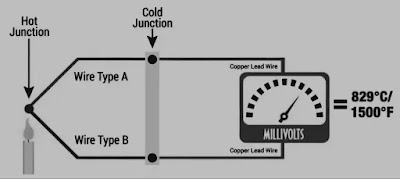
Thermocouple
A Thermocouple is a device that is used to measure temperature that instrument contains the joining of two dissimilar metals A and B. Seeback effect tells, a net e.m.f is generated in the circuit which depends on the difference in temperature between the hot and cold junctions. Microvoltmeter measures e.m.f. with a high degree of accuracy. The choice of metals varies depending upon their temperature range. Commonly copper-constantan, chromel-alumel, and platinum-platinum-rhodium are used.
At the 0-degree celsius reference junction, the thermocouple is calibrated by measuring e.m.f at various temperatures.
ɛ = a+bt+c t2+dt3
ɛ is thermal e.m.f. a,b,c, and d are constants it vary for different thermocouple.
That's all about the zeroth law of Thermodynamics and examples of the zeroth law of Thermodynamics.
I hope that you understand the definitions, and examples.
If you have any queries or doubts or need improvements in this article put that in the below-mentioned comments section we will reply and rectify as fast as possible.
Spark your brain and Throttle your knowledge!.



